Introduction
Continuous bioprocessing has emerged as a transformative approach in the production of biologics, including monoclonal antibodies (mAbs) and vaccines. Unlike traditional batch processing, continuous processes run uninterrupted over extended periods, enabling enhanced productivity, reduced footprint, and greater process consistency. However, this paradigm shift places unique demands on consumables and reagents, components critical for sustained and reliable operations.
What Is Continuous Bioprocessing?
Continuous bioprocessing refers to the seamless execution of upstream and downstream operations without discrete start-stop cycles typical of batch manufacturing. In this model:
- Upstream culture systems (e.g., perfusion bioreactors) continuously feed fresh media and harvest product.
- Downstream purification (e.g., chromatography and filtration) operates in a perpetual mode, processing incoming harvest in real time.
This continuous flow increases yield per unit volume and enhances process control. But success hinges on the reliable performance of consumables and reagents that support both upstream and downstream stages.
Why Consumables and Reagents Matter
Consumables and reagents may sound like “supporting actors,” but in continuous bioprocessing they play leading roles. Key reasons they are essential include:
- Process integrity: Continuous processes run for weeks to months; failure of a consumable (e.g., filter clogging) can halt the entire campaign.
- Product quality: Reagents and media directly impact cell health, yield, and product characteristics, including glycosylation profiles and aggregate levels.
- Operational efficiency: Durable, high-performing consumables reduce downtime and increase operational consistency.
- Regulatory compliance: Well-qualified reagents with traceable documentation support process validation and regulatory submissions.
Below, we delve into specific consumables and reagents used in continuous bioprocessing, categorized by their role in upstream and downstream operations.
Upstream Consumables and Reagents
1. Cell Culture Media and Supplements
At the heart of upstream processes lies cell culture media, a carefully formulated mix of nutrients, salts, and growth factors that sustain productive mammalian cell cultures (e.g., Chinese Hamster Ovary (CHO) cells).
- Basal media: Provide essential nutrients for cell growth.
- Feed solutions: Supplied continuously or periodically to maintain optimal nutrient levels.
- Supplements: Amino acids, vitamins, lipids, and growth factors to enhance productivity.
In perfusion systems, media and supplements must support high viable cell densities over extended periods. Media quality directly influences product yield and quality attributes.
2. Single-Use Bioreactors and Accessories
Continuous bioprocessing frequently leverages single-use technologies (SUTs), minimizing contamination risk and cleaning validation requirements.
- Single-use bioreactor bags: Replace traditional stainless steel systems; designed for weeks of perfusion culture.
- Connectors, tubing, and sensor probes: Sterile pathways for media, gasses, and sampling.
- Spares: In long-running processes, spare lines and connectors allow in-line change-outs without stopping.
SUTs enhance flexibility and reduce cross-contamination risks, but require robust quality control to ensure long term performance under continuous operation.
3. Cell Retention Devices
Perfusion systems rely on cell retention devices to separate cells from spent media while keeping them in the bioreactor.
Common technologies include:
- Filtration membranes: Tangential flow filtration (TFF) systems with durable membrane consumables.
- Spin filters: Mechanical filters that trap cells while allowing harvest flow.
- Alternating Tangential Flow (ATF): Uses diaphragms to generate cross flow.
Consumable membranes and filters must withstand continuous operation for weeks without fouling or failure.
4. Gas Supplies and Control Reagents
Maintaining the appropriate oxygen and carbon dioxide levels is pivotal for cell health.
- Sterile gas filters and spargers
- pH and dissolved oxygen control reagents
- Buffering agents
Reliable gas delivery systems and reagents that stabilize pH and gas exchange contribute significantly to culture stability and productivity.
Downstream Consumables and Reagents
Once product enters the downstream phase, consumables and reagents continue to be mission-critical.
1. Chromatography Resins and Columns
Continuous chromatography platforms, such as simulated moving bed chromatography or multicolumn setups, depend on premium resins:
- Protein A resins: Capture mAbs with high specificity.
- Ion exchange and mixed-mode resins: Polishing steps to remove impurities.
- Affinity resins: Critical for vaccine antigen purification.
In continuous mode, resins operate through many cycles, so stability and binding capacity are major performance metrics.
2. Filtration Devices
Filtration remains central in downstream workflows, including:
- Depth filters: Clarify crude feed streams.
- Ultrafiltration/diafiltration (UF/DF) membranes: Concentrate and buffer-exchange product streams.
Continuous filtration systems with robust consumables ensure consistent effluent quality and minimize fouling that could disrupt operations.
3. Buffers and Chemical Reagents
Downstream purification requires precise buffer compositions to optimize binding, elution, and product stability:
- Equilibration and wash buffers
- Elution buffers (often with pH modifiers or salts)
- Sanitization reagents (e.g., caustic solutions)
Reagents must be prepared and supplied in ways that support continuous delivery without compromising system sterility.
4. Inline Sensors and Probes
Real-time monitoring is fundamental to continuous processing:
- UV detectors (e.g., at 280 nm)
- Conductivity and pH probes
- Turbidity sensors
Consumables like sterile sensor housings and calibration standards help maintain data accuracy, enabling automated control loops for continuous purification.
Challenges with Consumables in Continuous Bioprocessing
Although continuous processing improves efficiency and quality, it introduces challenges:
1. Durability and Stability
Consumables must perform over extended run times without degradation. For example:
- Filters must resist fouling.
- Membranes must retain selectivity.
- Resins must withstand multiple cycles.
2. Supply Chain and Scalability
Long campaigns require large quantities of reagents and media. Supply continuity, inventory management, and lot-to-lot consistency become crucial risk factors for uninterrupted operations.
3. Validation and Regulatory Compliance
Consumables and reagents must be qualified and validated. This includes:
- Certificates of Analysis (CoAs)
- Material traceability and change control
- Compatibility with regulatory expectations for biologics production
These considerations become more complex as continuous systems integrate automated control strategies.
Trends and Innovations
The landscape of consumables and reagents is evolving. Innovations include:
1. High-Performance Resins
Next-generation chromatography media designed for extended cycle life and high dynamic binding capacity, reducing the need for frequent replacements.
2. Integrated Sensor Technologies
Smart probes with digital connectivity for advanced process control and predictive maintenance.
3. Modular Media Delivery Systems
Automated feed systems capable of preparing and delivering media and buffers on demand, minimizing manual intervention and error.
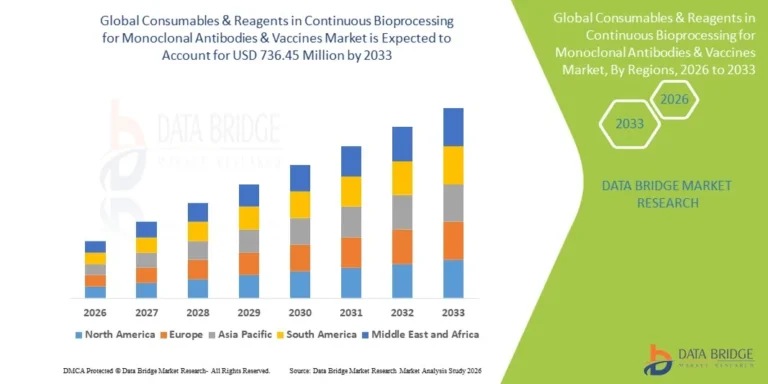
Growth Rate of Consumables and Reagents in Continuous Bioprocessing for Monoclonal Antibodies and Vaccines Market
According to Data Bridge Market Research, the consumables & reagents in continuous bioprocessing for monoclonal antibodies & vaccines market was estimated to be worth USD 392.04 million in 2025 and is projected to grow at a compound annual growth rate (CAGR) of 8.20% to reach USD 736.45 million by 2033.
Conclusion
Consumables and reagents are the unsung heroes of continuous bioprocessing for monoclonal antibodies and vaccines. Their performance influences everything from cell health and productivity to purification efficiency and product quality. Continuous processes demand durability, consistency, and scalability – requirements that place unique pressure on the consumables and reagents that enable them.